SoBliss®
The revolutionary sterile double-blister
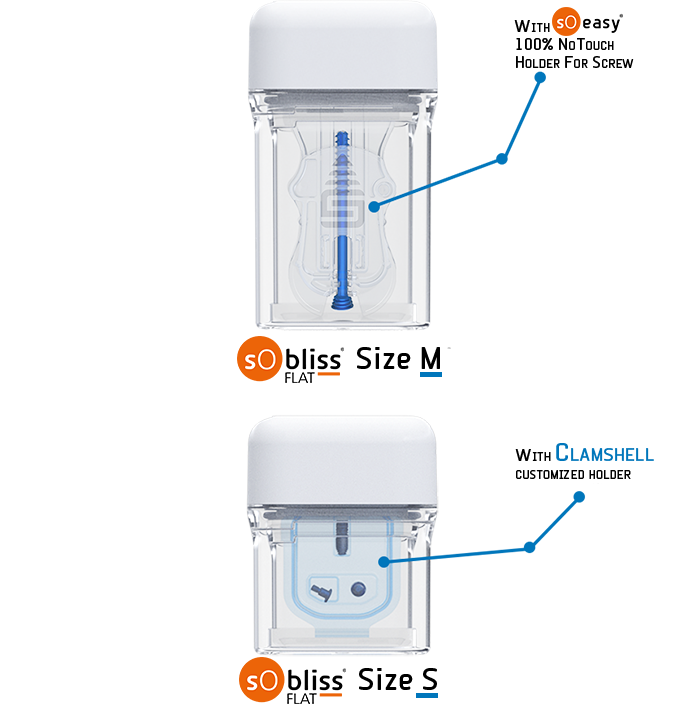
SoBliss® allows new blister packaging vision. Implants can now be packaged in 360° transparent & rigid “double-blister”, that provides a better match in terms of identification, handling, and storage. It also secures transfer procedure (no aseptic faults during surgeries thanks to its “No Touch” approach capability. SoBliss® is suited for a wide range of implants in a longitudinal and flat shape or slightly curved implants.
All raw materials have been analyzed and chosen for their ability to be used in the medical field to package or be in contact with medical device and are compliant with ISO 10993-1/5/11. SoBliss® Sterility can be maintained over a five years shelf life. Thanks to the hinge and vertical all-around sealing, a puncture roof Tyvek® system has been created.
Nurses panel interviewed appreciates the fast and self-intuitive opening system. (Tested during HEALTHPACK conference in March 2017 & AORN conference in March 2018 as preliminary usability study in USA)

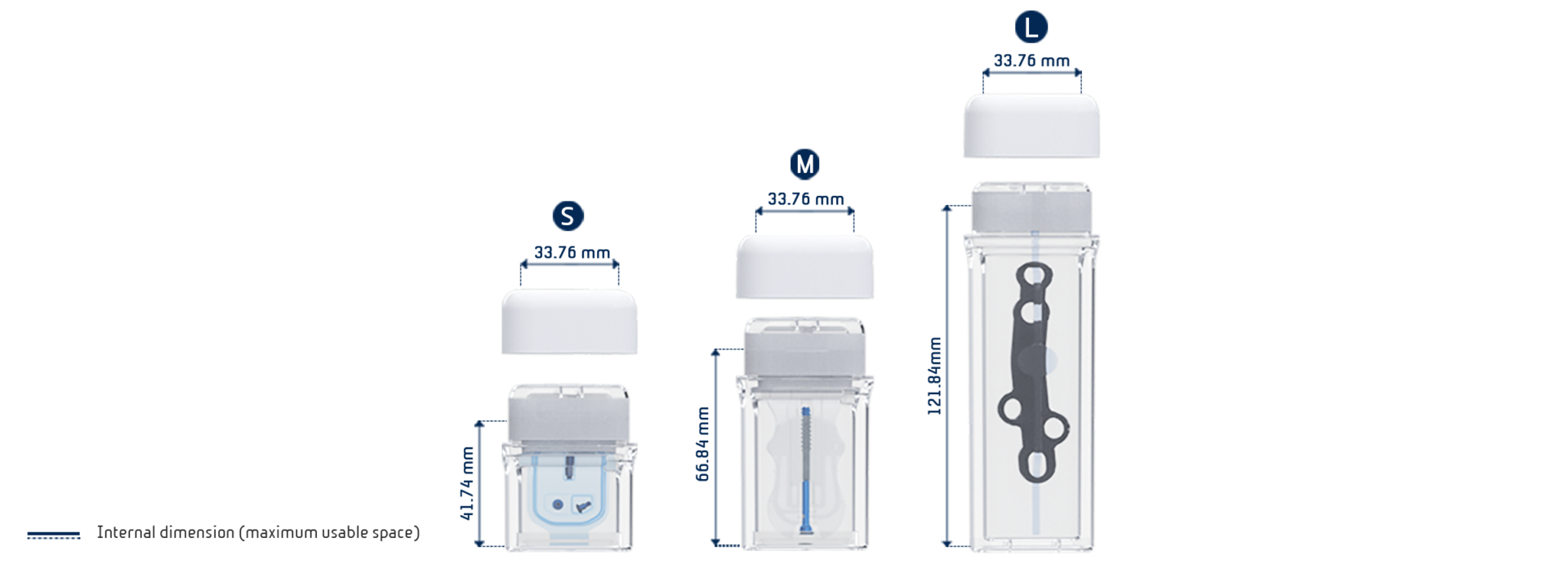
SoBliss® Performance:
- Easy storage and logistic cost savings all along the supply chain thanks to its reduced size
- Operating time reduced thanks to an optimized design (visual and control identification by the nurse, fast opening, secured transfer to the sterile side, etc.)
- Holding of the implant possible on a holder for a perfect hosting of the device in the SoBliss®
- SoBliss® range currently available in 3 sizes in double sterile configuration and 6 sizes in single barrier configuration
- Tyvek band peeling system highly appreciated by nurses, especially for its quick opening (Tested during HEALTHPACK conference in March 2017 and in a survey on more than 200 people during AORN in March 2018)
- Labels (patient and identification) directly attached to the packaging
- Single use with safety shrink wrap foil associated with its counterfeiting peeling strap
- Improved traceability
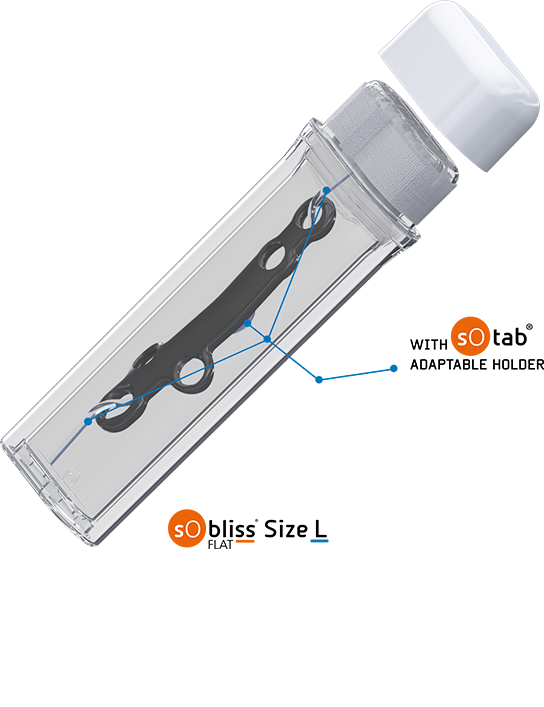
This packaging also secures transfer procedure and ensures no aseptic faults during surgeries thanks to its double-sterile barrier and its no touch capability. SoBliss® is therefore a new solution to reduce the estimated 2 million Hospital Acquired Infections (HAIs) contracted every year in U.S according the WHO. This number reaches 3 million HAIs in Europe. Additionally, about 80’000 patients in the U.S and 37’000 in Europe die every year following HAIs. The HAIs occurence can be lowered by setting up improvement in handling procedure. Studies show that this rate could be thus decreased by more than 70%.
Awards